In this briefing:
- RSNA launches a new AI journal.
- ACR makes several moves to advance the use of artificial intelligence in the future of medical imaging.
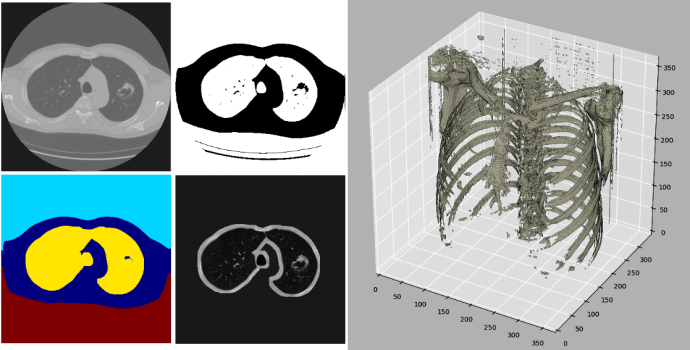
- Researchers publish in high impact journal the successful use of AI to detect breast density.
- ACR and MICCAI sign agreement to advance AI in medical imaging.
- Geisinger declares successful implementation of algorithm to improve time-to-diagnosis of intracranial hemorrhages 20-fold.
Stay up to speed in 2 minutes. Radiology AI Briefing is a semi-regular series of blog posts featuring hand-picked news stories and summaries on machine learning and data science.